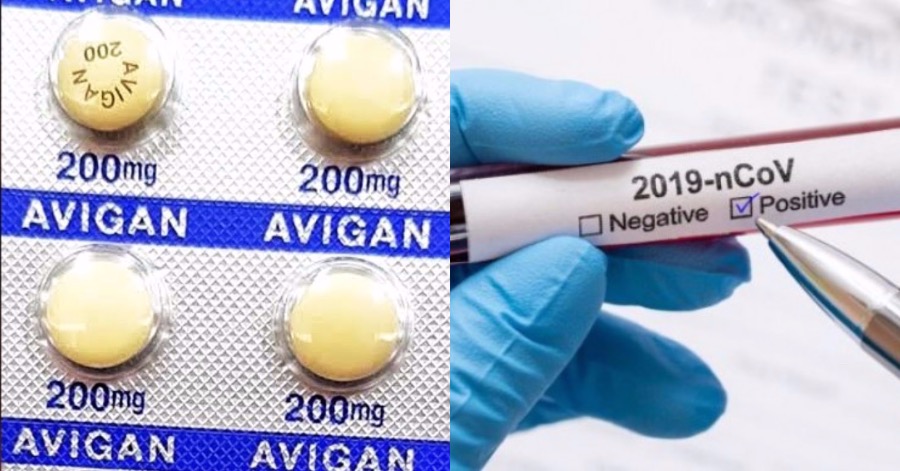
TOKYO, July 21 — A clinical trial for the influenza drug Avigan, a potential treatment for the COVID-19 disease caused by the novel coronavirus, will begin in Kuwait as early as this month, Jiji Press quoted informed sources Monday.
Up to 1,000 people are expected to participate in the trial to confirm the efficacy and safety of the drug as a coronavirus remedy.
Data collected through the trial may be used in applications for approval of the drug in Japan.
The trial will be led by Indian generic drugmaker Dr Reddy’s Laboratories Ltd, with whom Fujifilm Corp has signed a licensing contract for the overseas manufacturing and sales of Avigan.
The Japanese company will support the trial by supplying the drug.
Fujifilm Toyama Chemical Co, the Fujifilm subsidiary that developed Avigan, began a clinical trial in Japan in late March with a target of 96 participants.
The trial was slated to end in late June, but the end date has been pushed back due to a decline in the number of participants.
Source: BERNAMA








Leave a Comment