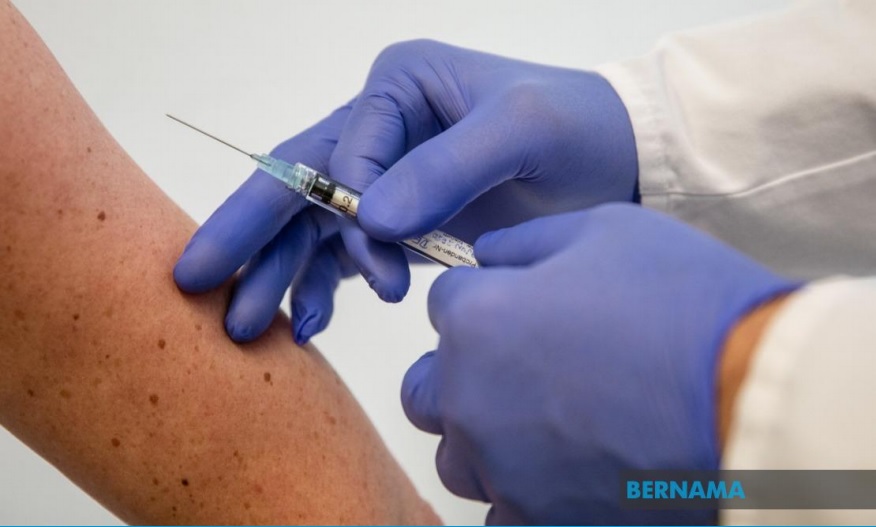
WASHINGTON, March 1 – Vaccine advisers of the United States Centres for Disease Control and Prevention (CDC) voted on Sunday to recommend the Johnson & Johnson COVID-19 vaccine for Americans 18 years of age and older.
The CDC’s Advisory Committee on Immunisation Practices is a group of vaccine and public health experts that help set CDC guidelines on vaccination practices, reported Xinhua news agency.
The recommendation came a day after the US Food and Drug Administration (FDA) authorised Johnson & Johnson’s COVID-19 vaccine for emergency use in the US on Saturday.
It is the third COVID-19 vaccine that has received the FDA’s emergency use authorisation in the country, following the first one developed by American drug maker, Pfizer in partnership with German company BioNTech, and the second one developed by American drug maker Moderna.
It is also the first single-dose COVID-19 vaccine available in the US.
Source: BERNAMA







Leave a Comment